Audioscan introduces the OTC (Over the Counter) / DTC (Direct to Consumer) Test Suite for Verifit2. By answering key questions, here is what you need to know about this new software feature.
1.What is the Audioscan OTC/ DTC Test Suite?
The Audioscan OTC/DTC Test Suite is a recent addition to the Verifit2 software, available in software version 2023.2.2. This new feature provides a means to assess the performance and audibility of OTC/DTC devices.
2. How does the Audioscan OTC/DTC Test Suite enhance clinical practice?
This new feature brings several advantages to clinical practice.
With the test results, clinicians can inform and counsel existing OTC device users about the quality and suitability of their devices. This opens the door for professional guidance, including prescribing hearing aids, especially if users are unsatisfied with their devices.
Additionally, it can measure the safety and utility of Personal Sound Amplification Products (PSAPs) and hearables, which are not FDA-regulated.
This tool can also aid clinics in expanding the range of technology options they offer. By confirming the safety and utility of the products, clinics can cater to a broader audience with varied needs and budgets.
3. What standards of OTC testing is this new feature based on?
The suite integrates the FDA's electroacoustic test battery, which is founded on consumer safety criteria. This safety criterion mandates manufacturers to collect and disclose a set of electroacoustic measurements and test results for all OTC products.
This standard relies on both the ANSI/CTA-2051 and ANSI S3.22 hearing aid measurement standards. It is important to note that this framework does not extend to Personal Sound Amplification Products (PSAPs) since they operate without FDA regulation, and compliance with ANSI/CTA-2051 standards is entirely optional.
The FDA consumer safety electroacoustic test battery and its associated performance criteria is embedded in the Audioscan OTC/DTC Test Suite.
4.How can I access the Audioscan Verifit 2 OTC/DTC Test Suite?
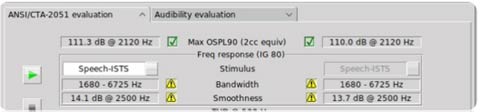
Verifit2 users can access these tests by selecting the OTC/DTC menu option, available in software version 2023.2.2 or later. The OTC/DTC Test Screen displays frequency response graphs, making it easy to evaluate performance. Once the ANSI/CTA-2051 Evaluation test sequence is completed the OTC/DTC will be given a green check mark if the device measured meets FDA ruling criteria per ANSI/CTA-2051 methods.
5.What styles of OTC devices can be verified?
Various OTC styles can be tested using different coupling techniques. For example, receiver-in-the-canal or thin tube-styled OTC devices can be coupled using TRIC adapters, while in-ear or earbud-styled devices can be coupled with blue putty.

6.What is the Audibility Evaluation feature?
The Audibility Evaluation is an added feature that is not mandated by the FDA. This test suite feature assesses if devices provide meaningful speech amplification for mild-to-moderate hearing loss, comparing their performance with established fitting targets, either the DSL 5.0a fitting rule or the NAL-NL2 fitting rule.
The Audioscan OTC/DTC Test Suite is a valuable tool for hearing health professionals, offering the means to verify the suitability of OTC devices, and provides sound evidence for the need to fit prescribed hearing aids.
7. Besides the OTC/DTC Test Suite, what other key features does Verifit2 offer compared to Verifit1?
-
Precise probe tube placement with ProbeGUIDE™
-
Enhanced efficiency with binaural real ear and test box capabilities, plus more...
Learn More about the Verifit 2 Hearing Aid Verification System here.
Download the latest software for your Verifit 2 system here.
To learn about the Audioscan OTC/DTC test suite, watch our interview with Dr. John Pumford of Audioscan now:
OTC Verification Q&A with Dr. John Pumford, Au.D. Part 1
OTC Verification Q&A with Dr. John Pumford, Au.D. Part 2
Other Good Reads: OTC Verification & The Future of Hearing Care: Q&A with Dr. John Pumford of Audioscan | Hearing Health & Technology Matters
Follow us on Social!


If you haven't already, make sure to subscribe to our newsletter to keep up-to-date with our latest resources and product information.